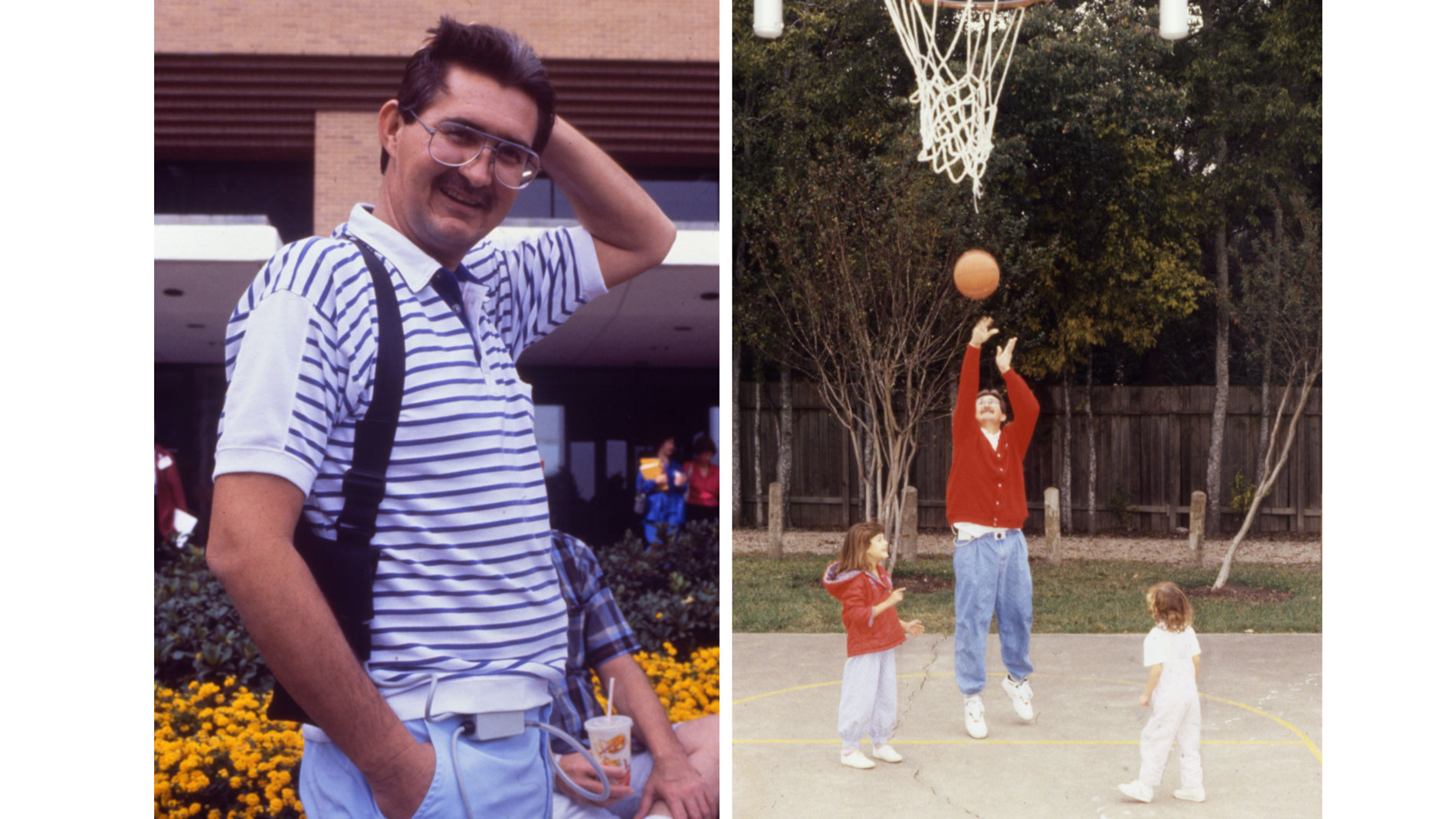History

Milestones of Texas Heart Institute's History
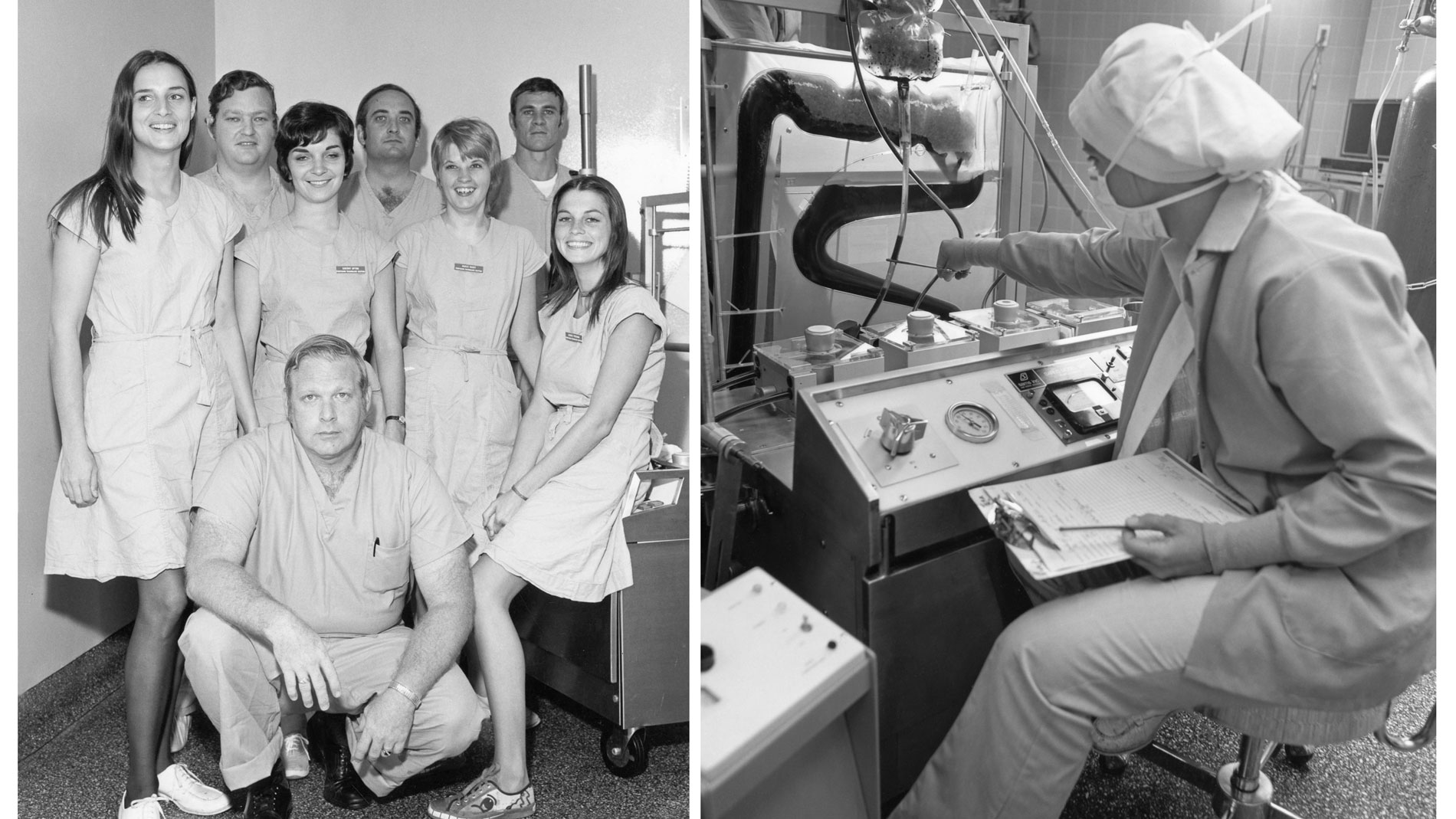
Dr. Cooley and his team had performed the first open-heart operations in Houston, and by 1962, SLEH and TCH had the most active open-heart surgical program in the world.
Ray C. Fish Foundation was established so that others might be encouraged to broaden man’s self-knowledge and to keep the American dream alive. After its founder’s death from heart disease, the Fish Foundation granted $5 million to make the Texas Heart Institute a reality. For this reason, the Institute’s highest professional award is given in honor of this extraordinary man. The award recognizes those whose innovations have made significant contributions to cardiovascular medicine and surgery. Ray C. Fish (1902-1962) was a leading figure in the natural gas industry and a philanthropist. Mr. Fish believed in the American dream of “opportunity for success.”
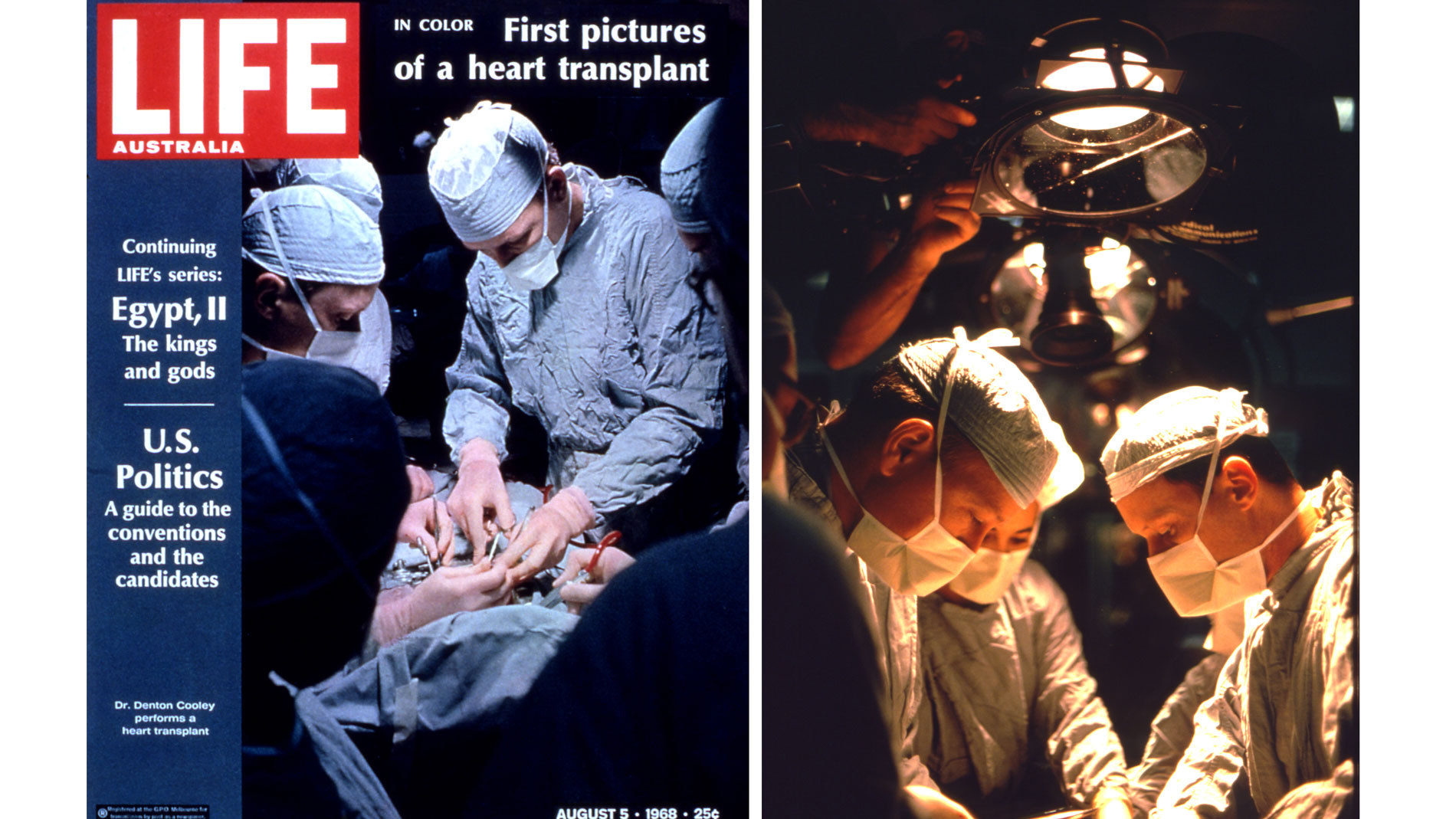
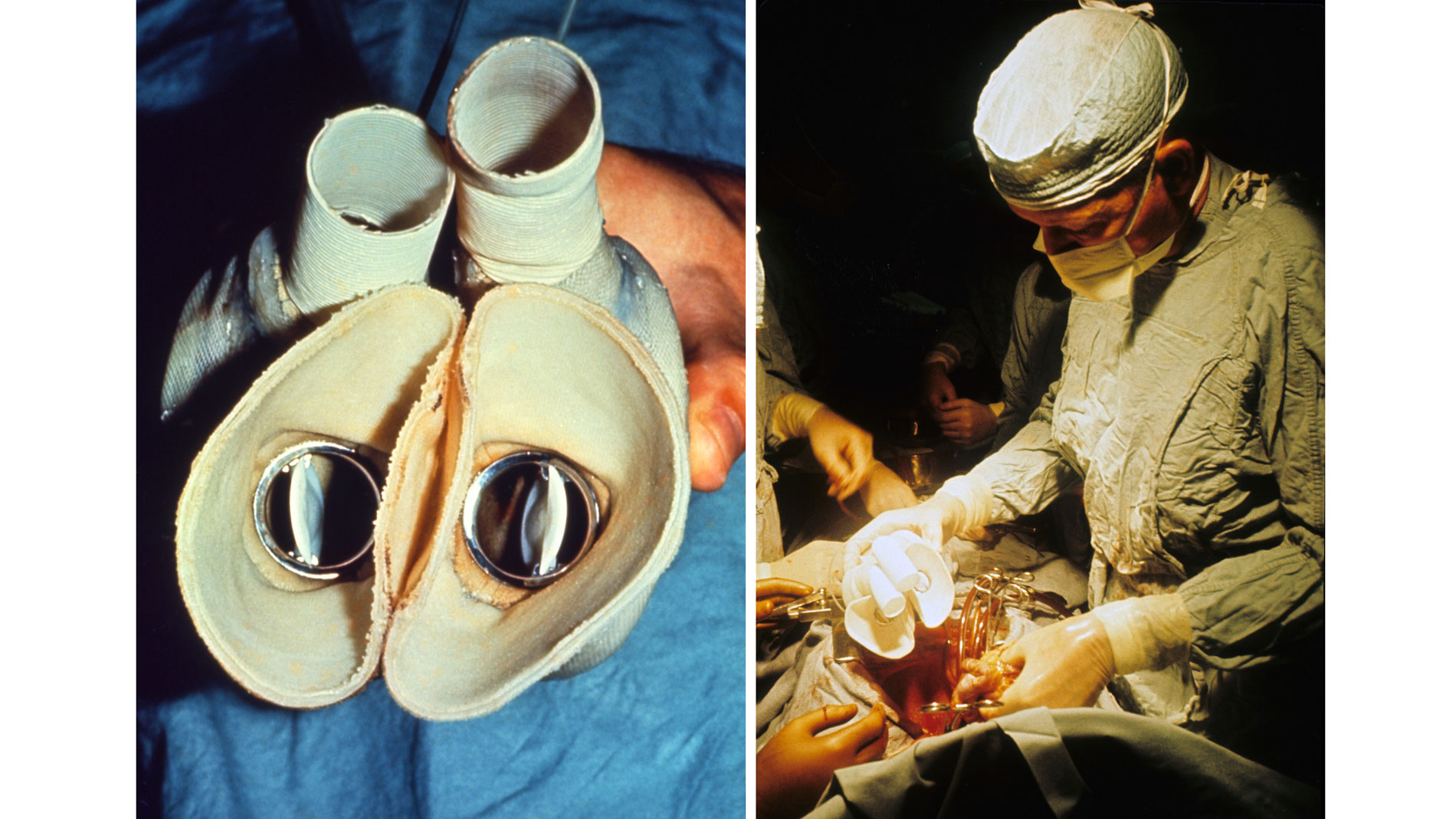
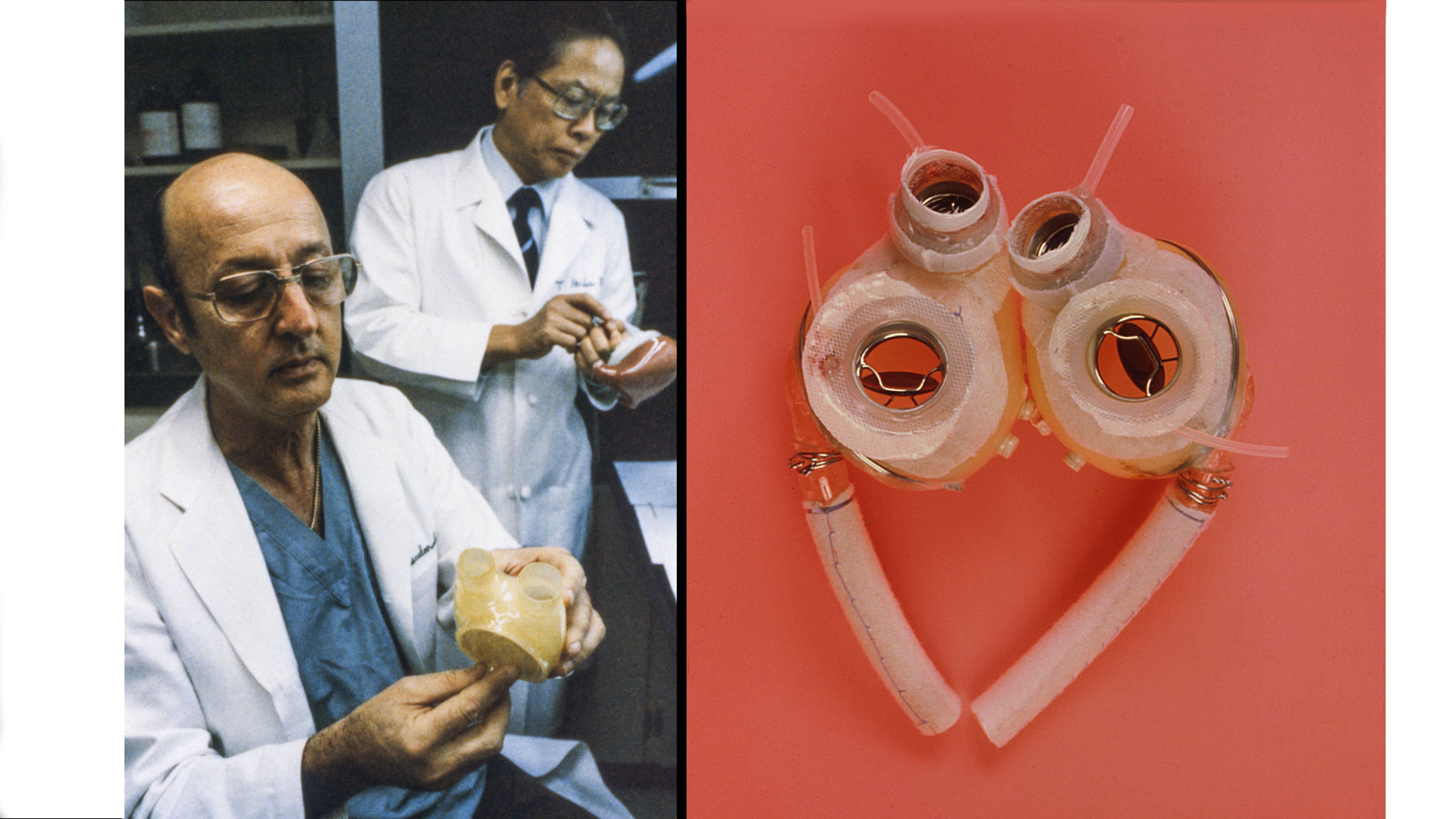
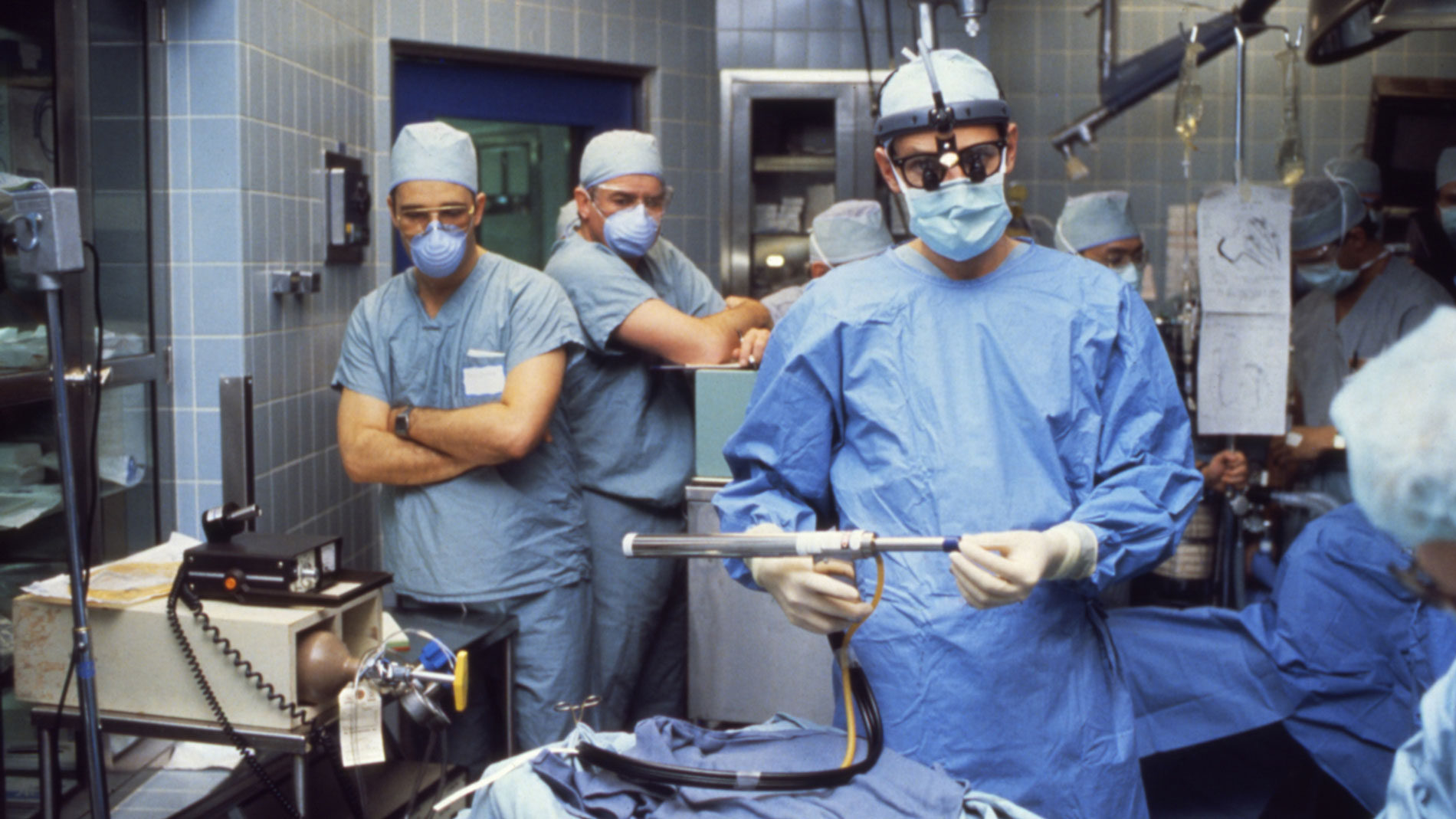
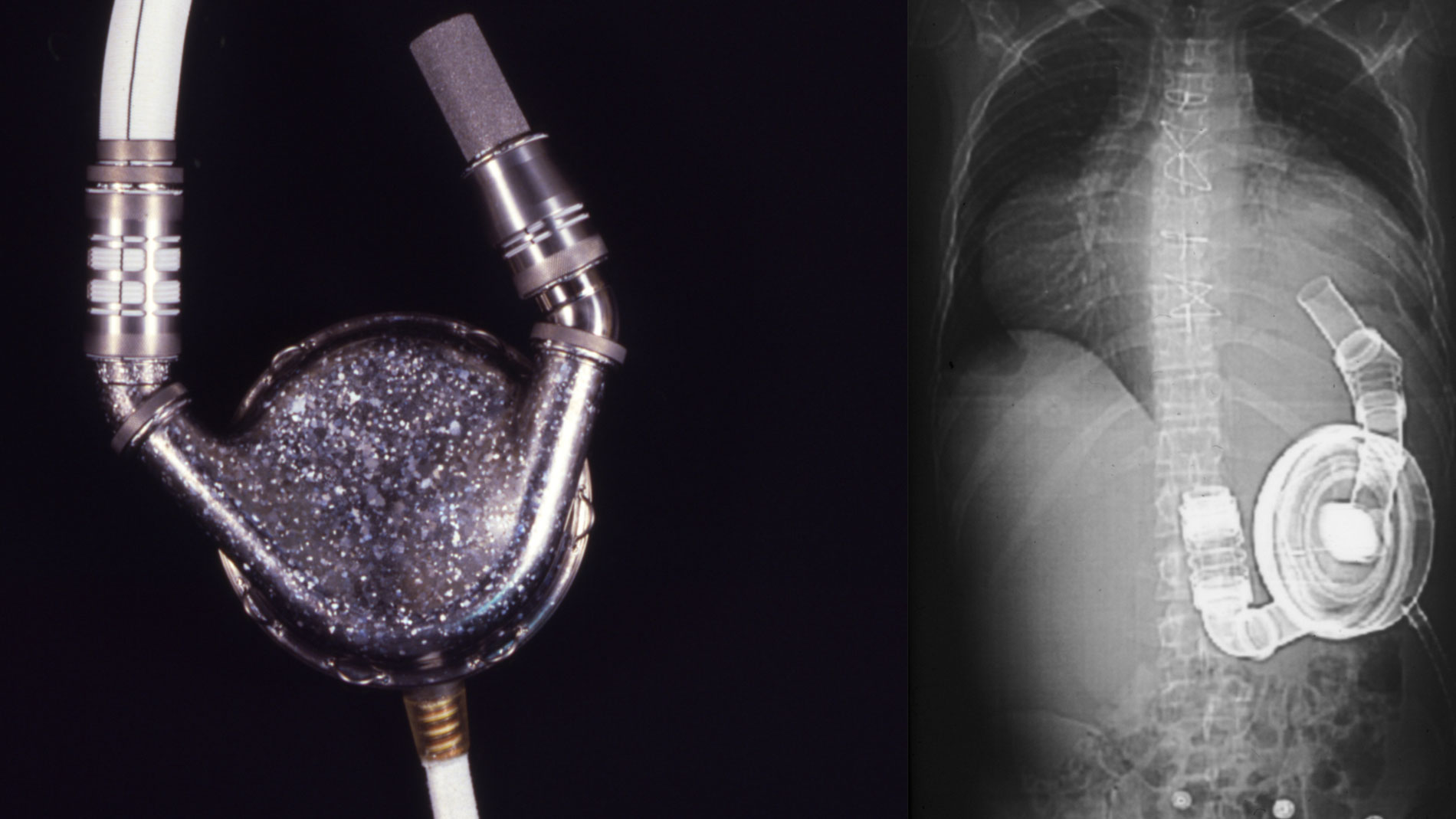
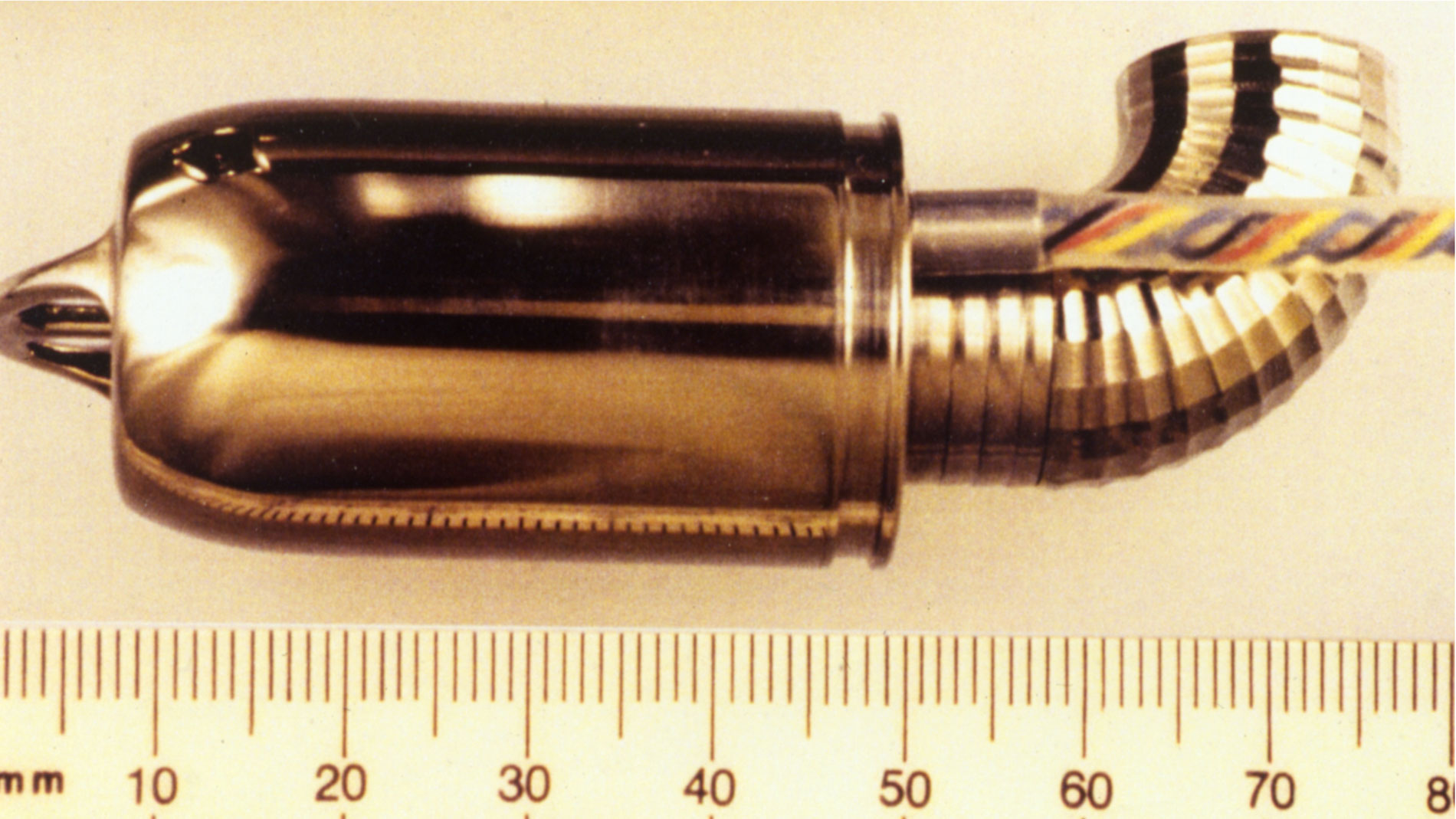
First implantation in the world of an artificial heart in a human. Dr. Denton A. Cooley performed the first total artificial heart implant in the world. The device, developed by Dr. Domingo Liotta, was implanted in a 47-year-old patient with severe heart failure. The Liotta heart supported the patient for nearly three days, at which time a donor heart was found for transplantation. This experience showed doctors that patients could be “bridged” to transplantation, meaning that mechanical circulatory support systems could be used to keep a patient alive until a donor heart is found. The Liotta total artificial heart was an air-driven (pneumatic), double-ventricle pump. Wada-Cutter hingeless valves controlled the flow of blood through the inflow and outflow areas of the pump. The two pump chambers (the “ventricles”), the cuff-shaped inflow tracts (the “atria”), and the outflow tracts were lined with a special fabric that promoted the formation of a smooth cellular surface. The flexible inflow and outflow tracts were made of Dacron fabric, and the pump chambers were made of Dacron™ fabric and Silastic™ plastic. (April 4, 1969)
First School of Perfusion Technology established in the US and remains one of the few accredited programs of its kind in the country.
The Cardiovascular Surgery Research (CVSR) Laboratories were established in 1972 to advance the understanding and treatment of cardiovascular disease. The labs have collaborated with government agencies and biomedical companies to conceive, design, test and apply new treatments, techniques and devices to improve patient care.
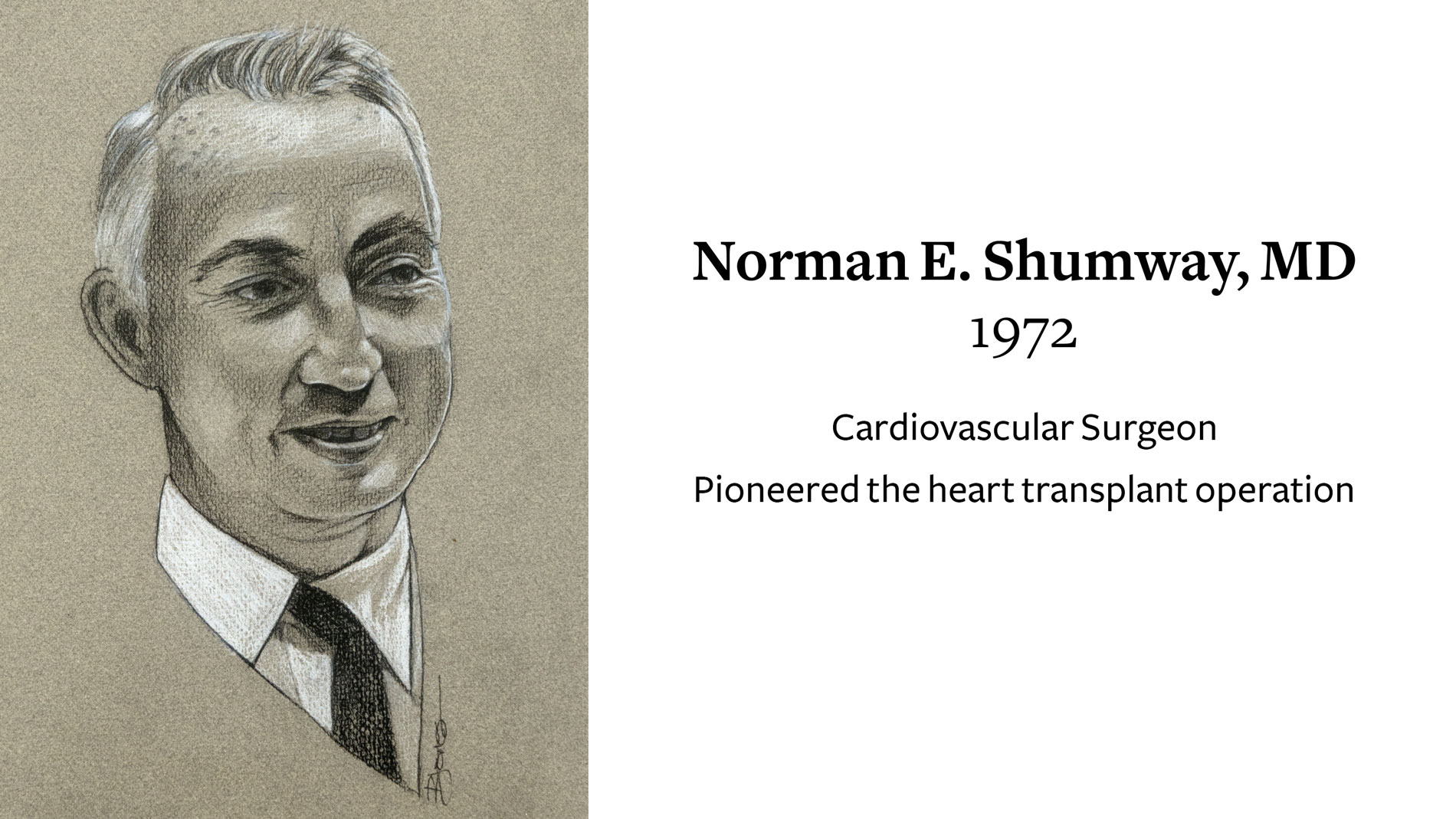
Norman E. Shumway, MD, Cardiovascular Surgeon, receives Ray C. Fish Award in recognition of pioneered the heart transplant operation.
F. Mason Sones, Jr., MD, Cardiologist, receives Ray C. Fish Award in recognition of advances made in the development of coronary angiography
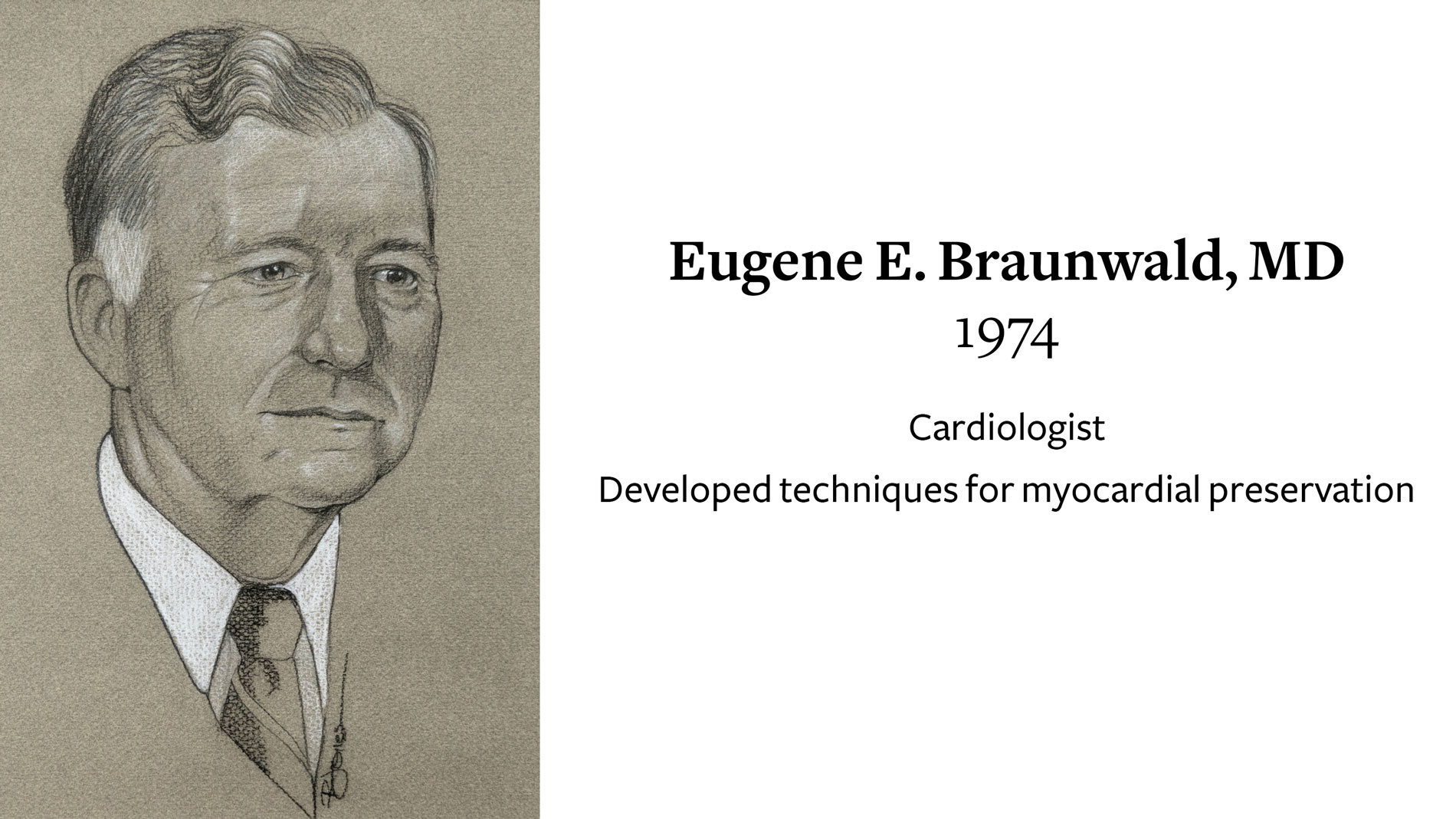
Eugene E. Braunwald, MD, Cardiologist, receives Ray C. Fish Award in recognition of development of techniques for myocardial preservation
First issue of the Texas Heart Institute Journal is published, under the name Cardiovascular Diseases: Bulletin of the Texas Heart Institute
Willem J. Kolff, MD, Cardiovascular Surgeon and Researcher, receives Ray C. Fish Award in recognition of his work in developing artificial organs
First study funded by the National Heart, Lung, and Blood Institute of an implantable LVAD for postcardiotomy support
Harvey Feigenbaum, MD, Cardiologist, receives Ray C. Fish Award in recognition of development of echocardiography
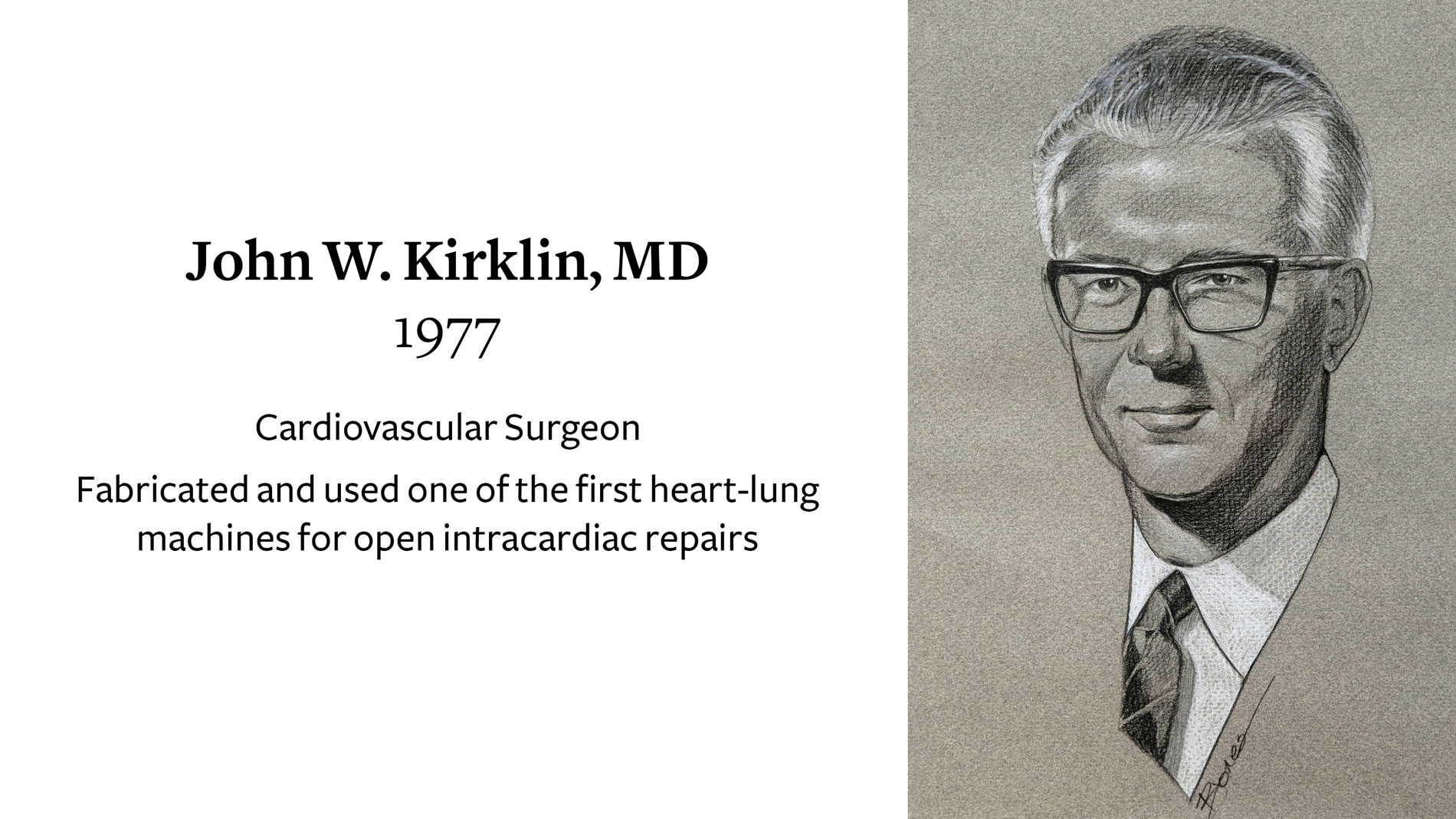
John W. Kirklin, MD, Cardiovascular Surgeon, receives Ray C. Fish Award in recognition of fabrication and use of one of the first heart-lung machines for open intracardiac repairs
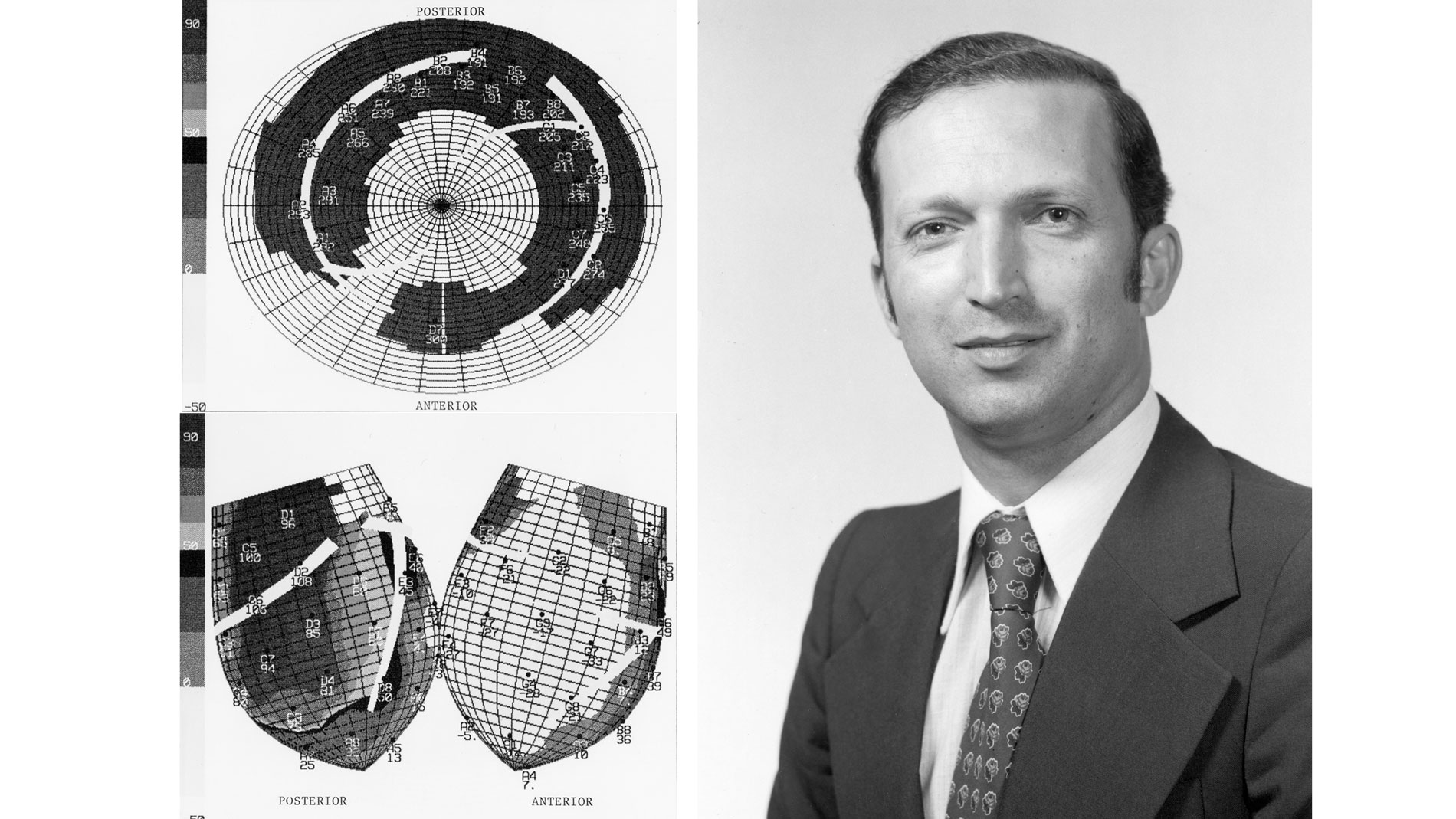
Bernard Lown, MD, Cardiologist, receives Ray C. Fish Award in recognition of pioneering the use of devices, including the defibrillator and cardioverter, to regulate cardiac rhythm disturbances
John J. Gallagher, MD, Cardiologist, receives Ray C. Fish Award in recognition of his work with Dr. Will Sealy to develop methods to correct Wolff-Parkinson-White (pre-excitation) syndrome
Will C Sealy, MD, Cardiovascular Surgeon, receives Ray C. Fish Award in recognition of his performance of the first successful surgery for Wolff-Parkinson-White (pre-excitation) syndrome
W. Proctor Harvey, MD, Cardiologist, receives Ray C. Fish Award in recognition of his renowned ability to diagnose and treat heart disease and for his ability to teach
Paul M. Zoll, MD, Cardiologist, receives Ray C. Fish Award in recognition of discoveries that led to the development of pacemakers
Dr. Denton A. Cooley again implanted a total artificial heart, the second such procedure in the world. Developed by Dr. Tetsuzo Akutsu at the Texas Heart Institute, the Akutsu III total artificial heart was implanted in a 36-year-old man. The Akutsu III kept the man alive for 55 hours, until a donor heart was found for transplantation. (July 1981)
Andreas R. Grüntzig, MD, Cardiologist, receives Ray C. Fish Award in recognition of scientific advances made in the development of percutaneous transluminal coronary angioplasty (PTCA)
Hein J.J. Wellens, MD, Cardiologist, receives Ray C. Fish Award in recognition of his discovery of reproducible methods for studying cardiac rhythm disturbances and the effects of various therapies on arrhythmias
Douglas P. Zipes, MD, Cardiologist, receives Ray C. Fish Award in recognition of his renowned reputation for treating cardiac arrhythmias, including sudden cardiac death
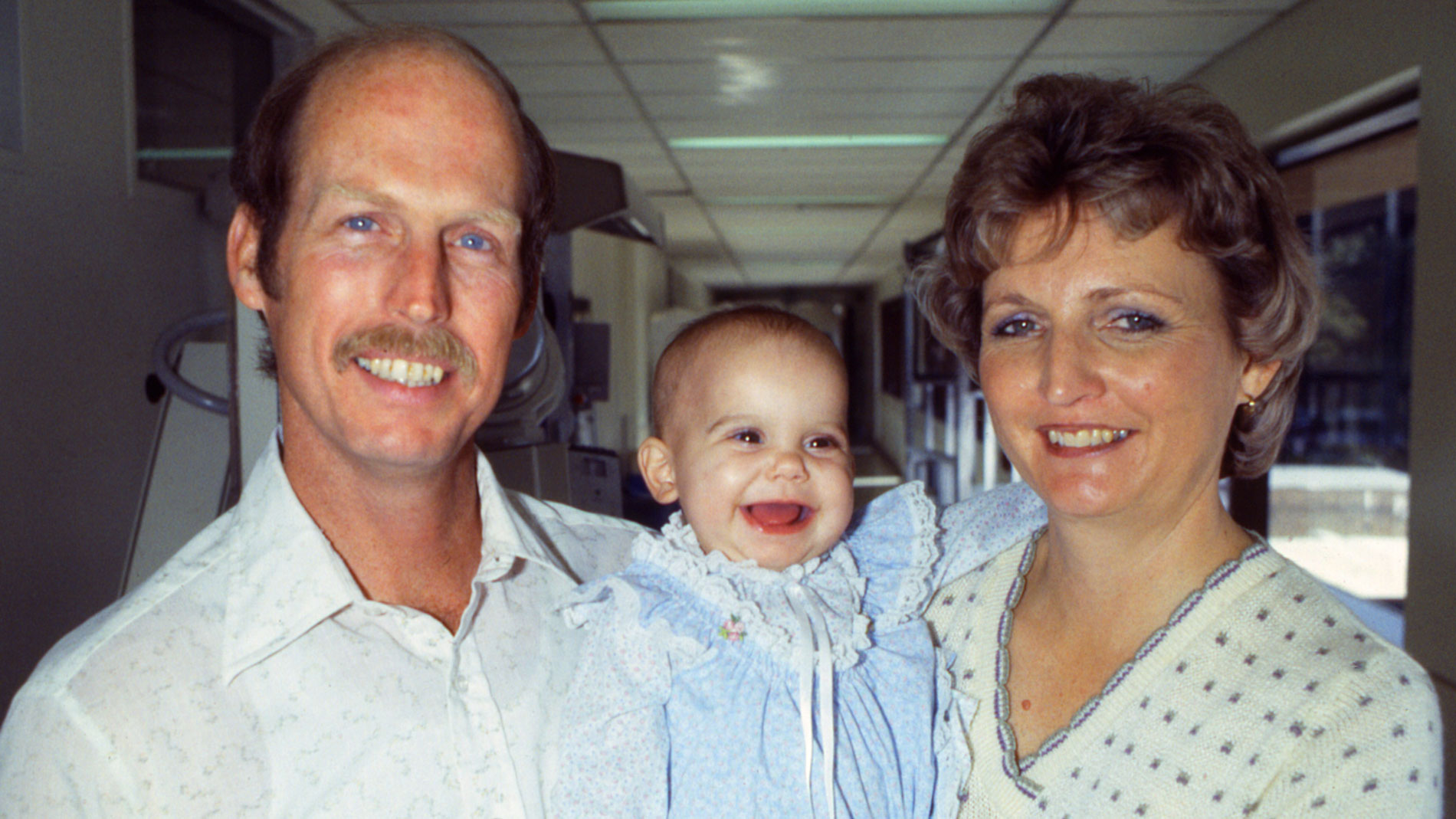
Dr. Denton Cooley and Dr. Howard Frazier performed heart transplant surgery on a 6-month old patient, Sara Remington at THI. She received the heart of a 3-year-old accident victim.
Leading with the Heart: by 1997, Sara Remington was the longest-surviving pediatric heart transplant recipient in the world. Although life expectancy is usually short for young transplant patients, Sara lived a healthy life and enjoyed a normal childhood.
Denton A. Cooley, Cardiovascular Surgeon, receives Ray C. Fish Award for pioneered numerous surgical treatments for heart disease, including implantation of a total artificial heart, heart transplantation, repair of left ventricular and aortic aneurysms, and palliation of congenital heart disease
William J. Rashkind, MD, Pediatric Cardiologist, receives Ray C. Fish Award in recognition of the development of a nonsurgical method to treat transposed heart vessels in infants
Dr. O.H. Frazier of the Texas Heart Institute (THI) initiated clinical trials for the Thoratec HeartMate IP LVAS (implantable pneumatic). THI was the first center to take part in these studies, and its surgeons and researchers played a key role in establishing that the pumps could sustain patients with heart failure. In October 1994, final marketing approval was received from the US Food and Drug Administration. Today, the Thoratec HeartMate IP LVAS is used worldwide as a bridge to heart transplantation. The HeartMate can greatly improve the clinical status of bridge-to-transplant patients. Most patients who are in New York Heart Association Functional Class IV (very severe heart failure) before receiving the HeartMate can improve to Class I while supported by the device. Meanwhile, they can be rehabilitated physically. When patients are supported by the HeartMate for more than 30 days, the outcome of transplantation improves.
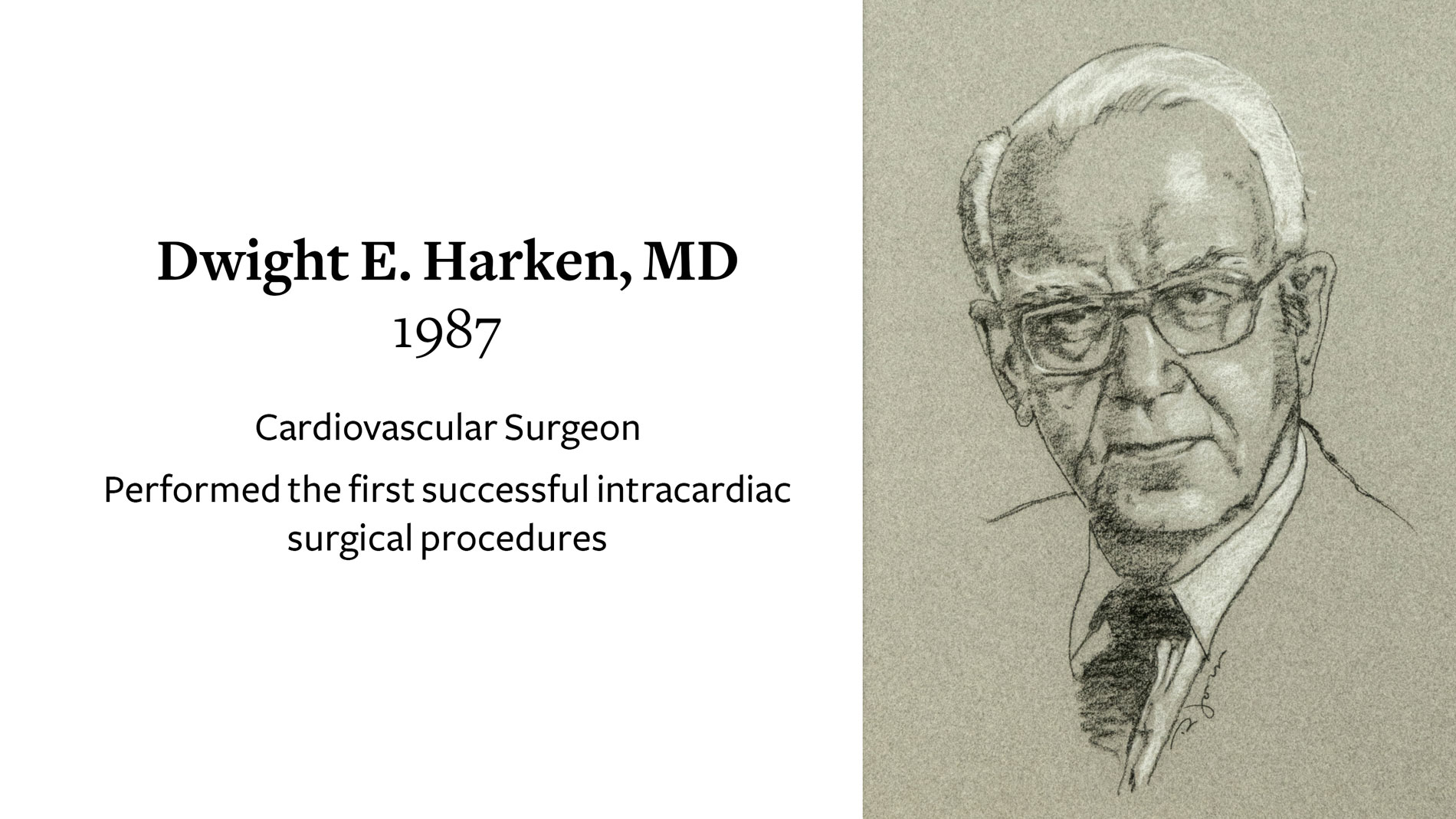
Dwight E. Harken, MD, Cardiovascular Surgeon, receives Ray C. Fish Award in recognition of performing the first successful intracardiac surgical procedures
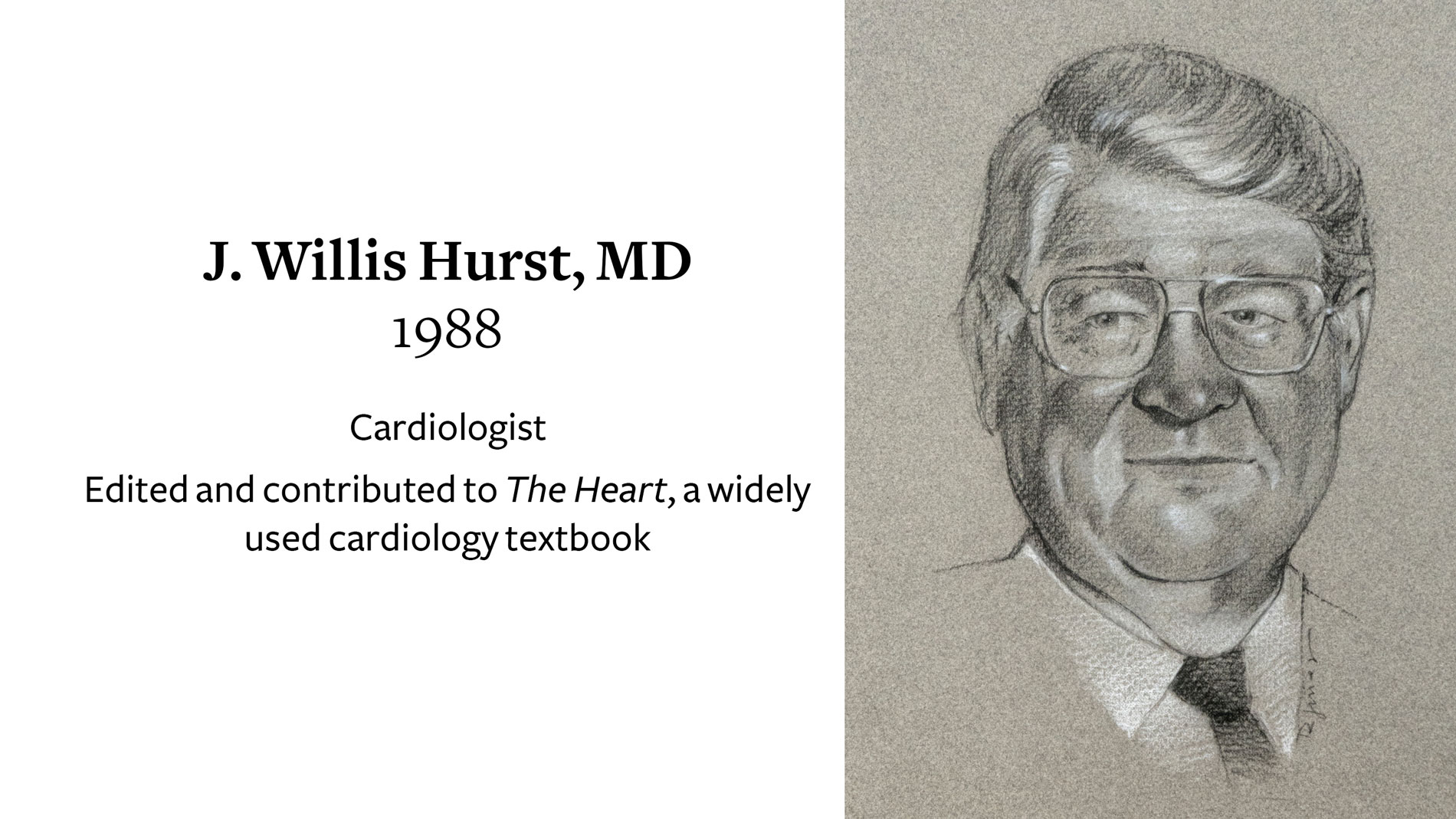
J. Willis Hurst, MD, Cardiologist, receives Ray C. Fish Award in recognition of his editorial contribution to The Heart, a widely used cardiology text
First heart assist device approved by the US Food and Drug Administration for the support of post-cardiotomy patients (those who have developed heart failure as a result of heart surgery). The ABIOMED BVS 5000™ is used worldwide for temporary left, right, or biventricular (both ventricles) support in patients with potentially reversible heart failure. The BVS 5000 underwent preclinical studies at the Texas Heart Institute (THI) from 1986 to 1988 and was introduced for use in patients at THI in 1988.
Jarvik Heart, Inc. and the Texas Heart Institute began developing the Jarvik 2000 Heart in 1988. About the size of a “C” battery, the device is a valveless, electrically powered axial flow pump that fits directly into the left ventricle and continuously pushes oxygen-rich blood throughout the body. In April 2000, THI was granted permission by the Food and Drug Administration to evaluate the Jarvik 2000 Heart as a bridge to transplantation in five patients. In October 2000, the FDA gave permission to extend the study. Patients have been sustained for more than 400 days with this device.
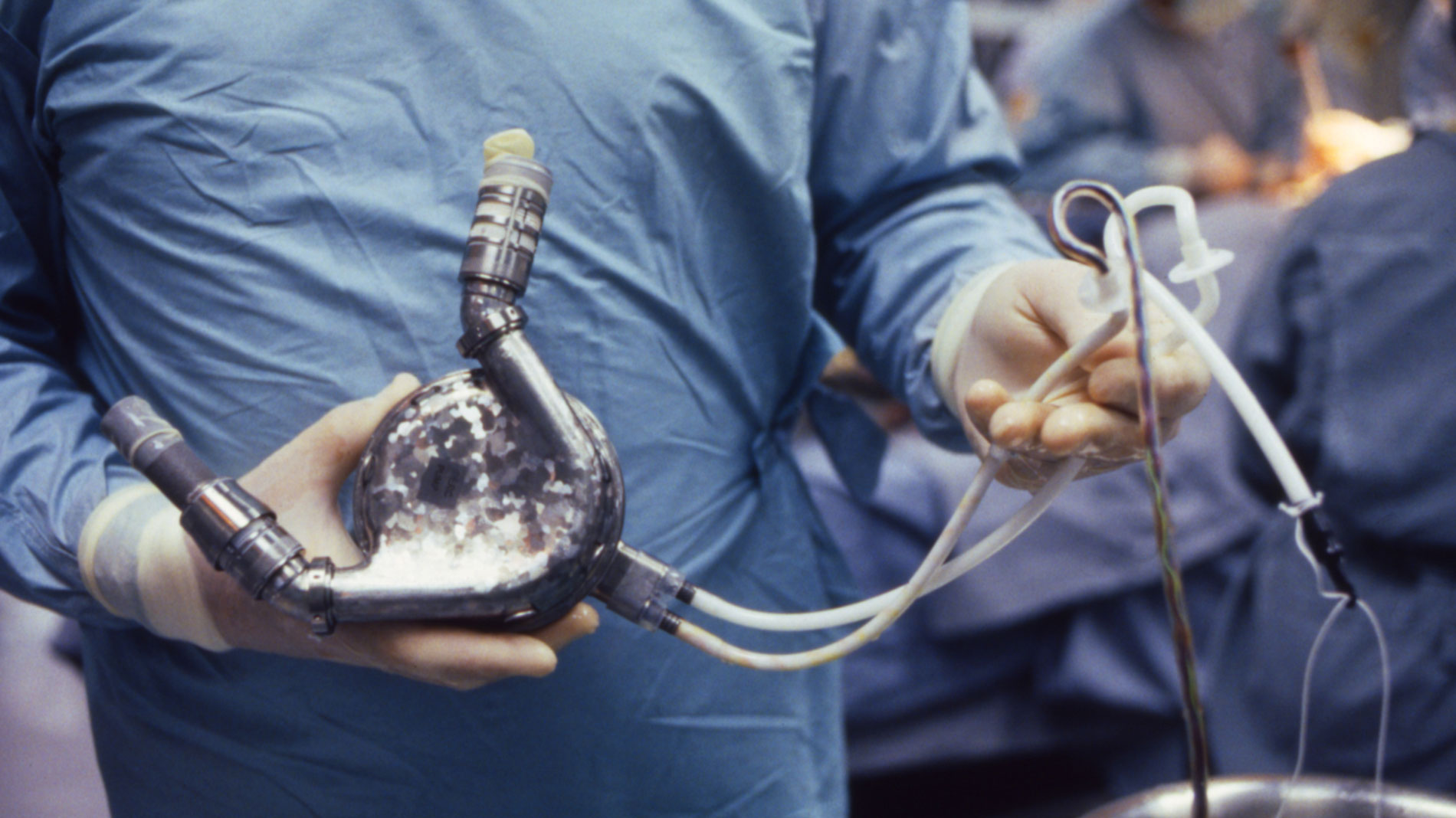
Clinical use of the Hemopump in patients began at the Texas Heart Institute as a short-term treatment (hours to days) for cardiogenic shock. Later, the device was evaluated as an alternative to standard cardiopulmonary bypass. Today, the Hemopump is no longer used, but researchers have applied its design to other circulatory assist devices. The innovative design of the Hemopump included a tiny axial flow pump that provided up to 3.5 liters per minute of circulatory support. The first patient treated with the Hemopump was a 61-year-old man with profound heart failure related to allograft (donor heart) rejection. His life was sustained with the Hemopump for two days, and he was eventually discharged from the hospital.
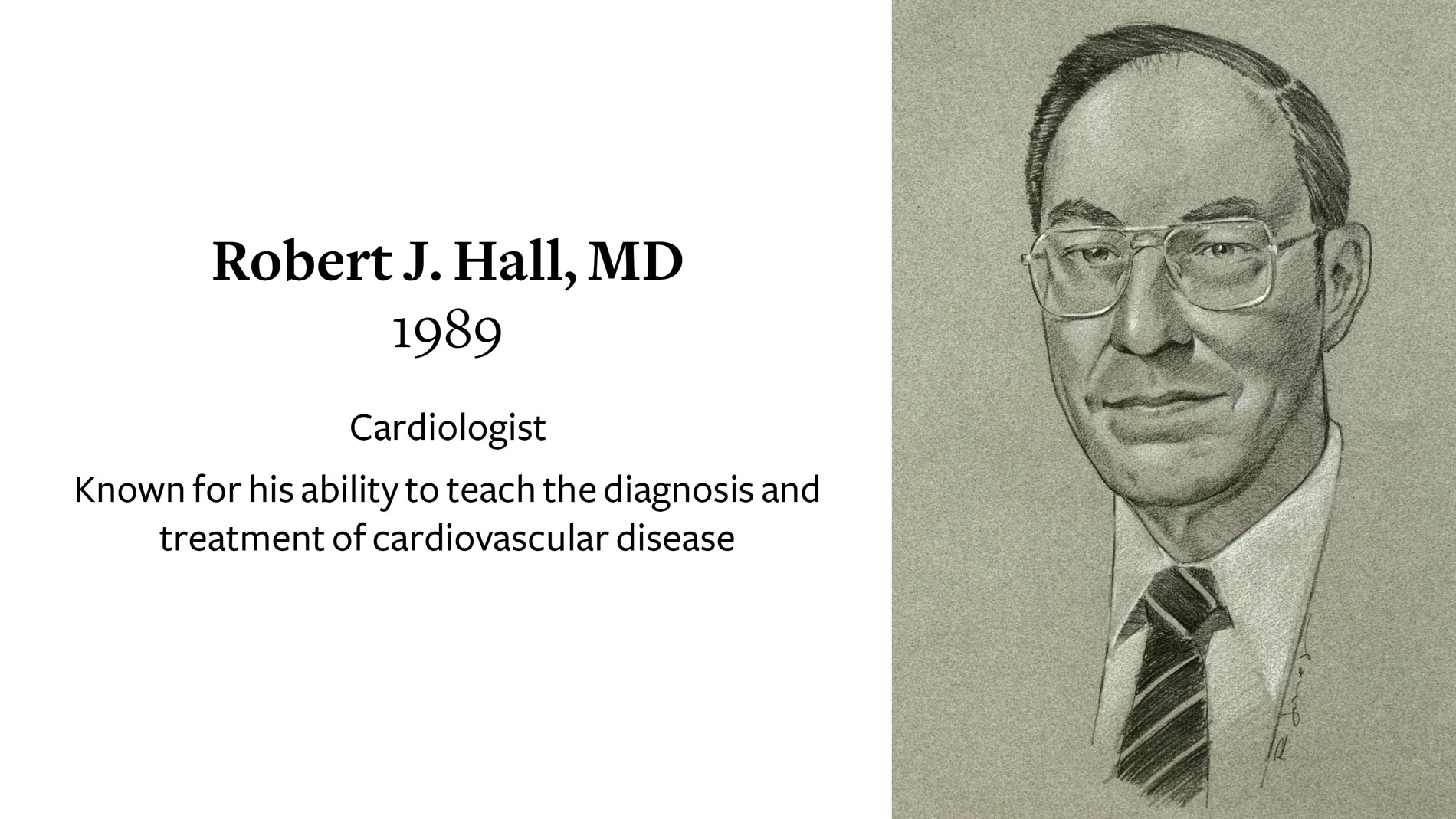
Robert J. Hall, MD, Cardiologist, receives Ray C. Fish Award in recognition of his ability to teach the diagnosis and treatment of cardiovascular disease
Late 1980s, surgeons at 16 centers, including THI, had used the Jarvik 7 as a bridge to heart transplantation in more than 70 patients.
Sol Sherry, MD, Cardiologist, receives Ray C. Fish Award for pioneered the use of thrombolytic therapy to treat blood clots
First cases of MAZE surgery performed at St. Luke’s Episcopal Hospital for atrial fibrillation.
The Thoratec HeartMate® XVE LVAS (vented electric) was developed and tested by Thermo Cardiosystems, Inc. and the Texas Heart Institute. In 1991, the system was implanted in a patient who was subsequently supported for 505 days.
This patient, who was able to leave the hospital while being supported by this device, paved the way for other patients to routinely wait at home for their transplants. Because the system was relatively easy to use, patients and their families could maintain it outside the hospital setting. Patients were now able to live at home, return to work, and resume a more normal lifestyle while awaiting a suitable donor heart. The Food and Drug Administration (FDA) approved the HeartMate XVE LVAS for destination therapy, which means the device could be used as permanent support for end-stage heart failure patients not eligible for heart transplants. (Journal of Thoracic Cardiovascular Surgery) (NEJM)
Arthur S. Keats, MD, Cardiovascular Anesthesiologist, receives Ray C. Fish Award in recognition of development of methods to make heart surgery safer for children and adults
THI selected as one of 7 centers in the United States to participate in the federal government’s Heart Demonstration Project for Medicare patients
American Heart Association journal Circulation placed at THI/SLEH for an 11-y period, the second longest tenure of placement in the journal’s history, with Dr James Willerson as Editor-in-Chief
Dr. James T. Willerson, named Editor-in-Chief of the American Heart Association journal Circulation. As the longest-serving Editor-in-Chief of Circulation, his tenure lasted 11 years.
Aldo R. Castaneda, MD, PhD, Pediatric Cardiovascular Surgeon, receives Ray C. Fish Award in recognition of Advocated early (neonatal) repair of complex congenital heart defects
Julio C. Palmaz, MD, Radiologist, receives Ray C. Fish Award in recognition of the invention of the first successful intravascular stent
The Texas Heart Institute began using the Thoratec VAD. One or two Thoratec VADs can be used to provide left, right, or biventricular support. The blood pump is positioned outside the body (extracorporeally) and connected to tubes (cannulas) inserted into the heart. The pump has a rigid plastic case that contains a flexible pumping sac. Blood is ejected from the pump when the pumping sac is compressed by air from the external control console. Within the inflow and outflow conduits, mechanical valves control the direction of blood flow. The Thoratec VAD has a stroke volume of 65 milliliters. It can be operated at up to 100 beats per minute, resulting in blood flow rates of up to 7 liters per minute.
Sir Magdi Yacoub, FRCS, Cardiovascular Surgeon, receives Ray C. Fish Award for pioneering work in heart–lung transplantation surgery
Groundbreaking for the new Texas Heart Institute at St. Luke’s Episcopal Hospital—The Denton A. Cooley Building.
FDA approval of a stent graft tested exclusively at THI/SLEH for repair of abdominal aortic aneurysms.
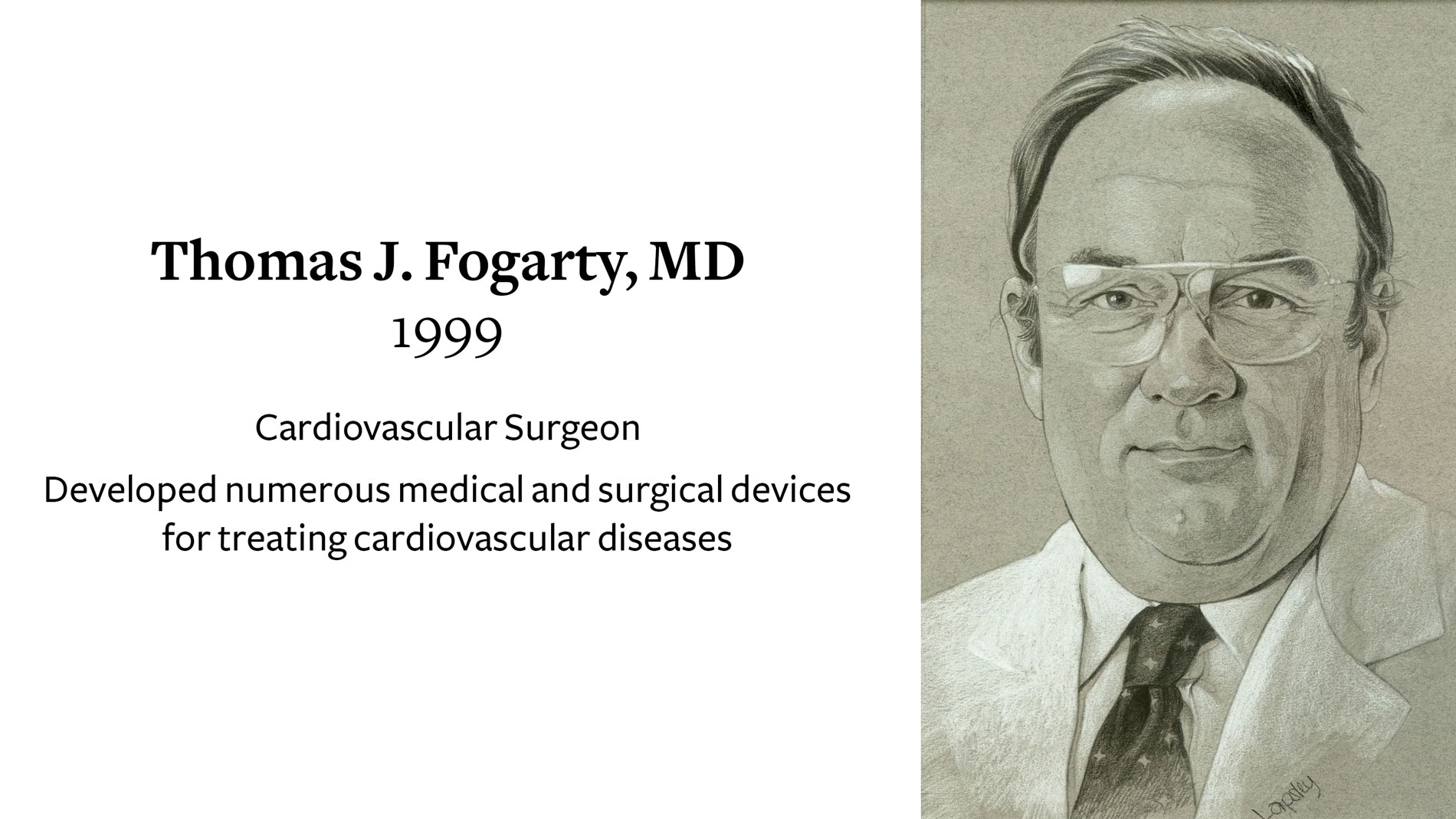
Thomas J. Fogarty, MD, Cardiovascular Surgeon, Cardiovascular Surgeon, recieves the Ray C. Fish Award for developing numerous medical and surgical devices for treating cardiovascular diseases
First site for clinical trials of the Jarvik 2000, a miniature, axial flow left ventricular assist device.
Completed REMATCH study, which compared long-term implantation of the HeartMate electric LVAD to conventional medical therapy for heart failure.
First in the world to treat coronary heart disease patients with heart failure and no other treatment options with their own stem cells by directly injecting them into the heart with a NOGA catheter
THI researchers began pioneering the use of stem cells for the treatment of heart failure and coronary heart disease in patients with no other treatment options. Dr Perin led the first clinical study to test the safety and efficacy of treating heart failure patients with coronary heart disease and no other treatment options with their own bone marrow–derived stem cells by using a NOGA catheter, which allows the physician to identify and inject the cells directly into reversibly injured endocardium. The success of this study paved the way for larger, randomized clinical trials of stem cell therapies.
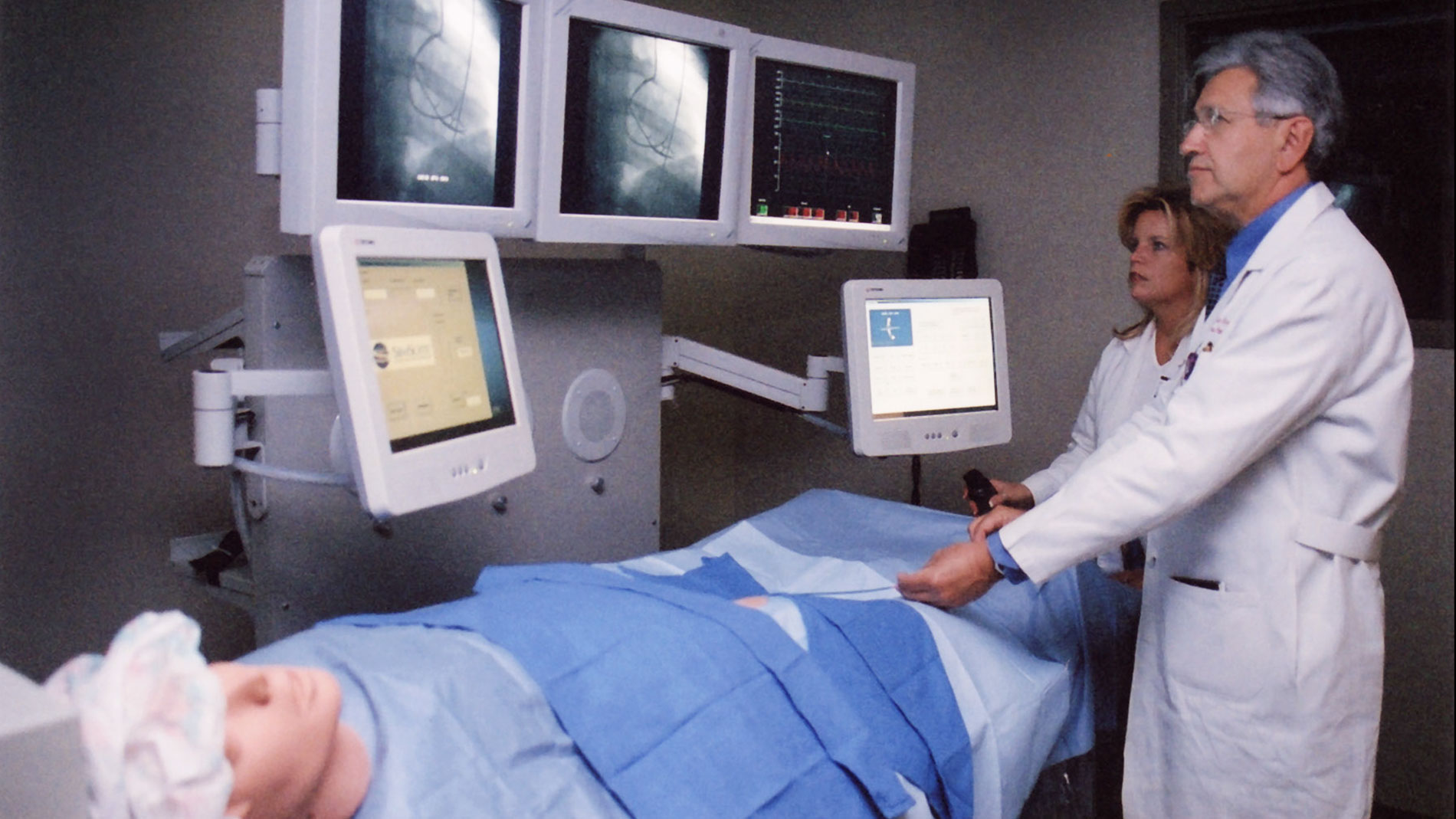
First nationally ranked cardiovascular center in the United States to open a cath lab medical simulation training center.
The ABIOMED AB5000™ Circulatory Support System was introduced for patient use at THI in October 2003. This short-term mechanical system provided left, right, or biventricular support for patients whose hearts have failed but have the potential for recovery. The AB5000 is used to support the heart, giving it time to rest—and potentially recover native heart function. The device can also be used as a bridge to definitive therapy.
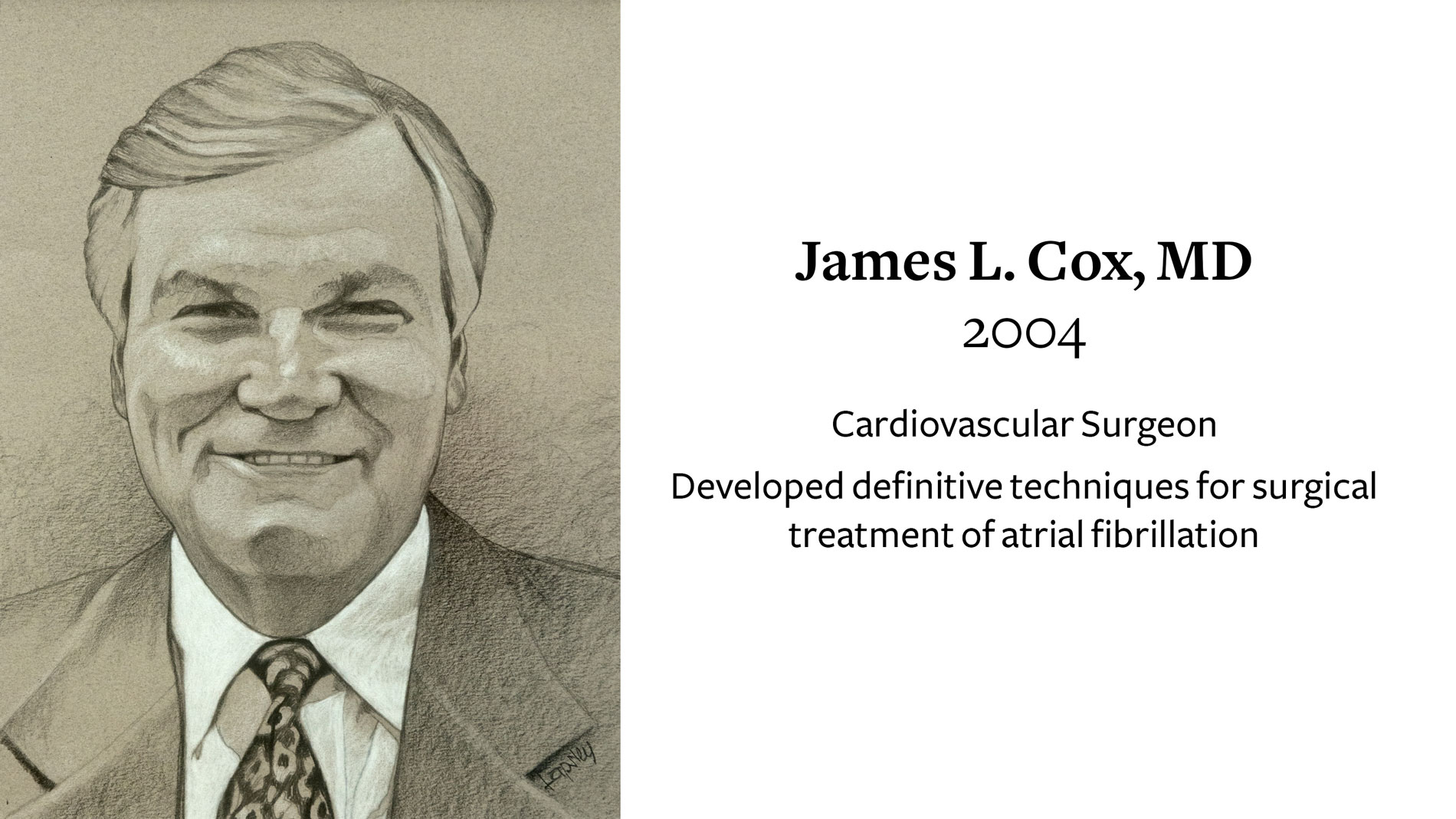
James L. Cox, MD, Cardiovascular Surgeon, receives Ray C. Fish Award for developing surgical techniques to definitively treat atrial fibrillation
Stephen Westaby, BSc, MS, PhD, FRCS Cardiovascular Surgeon, receives Ray C. Fish Award in recognition of his significant contributions in initiatiing the first clinical trial of axial-flow devices for destination therapy and significant contributions made to the scientific literature based on his extensive surgical experience.
First FDA-approved clinical trial of autologous adult stem cell therapy for congestive heart failure in the United States
THI and the University of Texas System sign a legal affiliation agreement to collaborate in cardiovascular disease research and education and the recruitment of outstanding physician-scientists
Project Heart website announced, providing a heart-smart resource for teachers in grades K through 6.
Dr Cooley’s 87th birthday, he performed his last surgical operation. At first, he missed the operating room greatly, but, as an octogenarian, he believed that it was time to step aside.
Dr. Charles Mullins, receives 2007 Ray C. Fish Award in recognition of his dedication to teaching and his pioneering work in developing interventional techniques for treating congenital heart., Receives Ray C. Fish Award for
Scientific Achievement
HeartMate II® Left Ventricular Assist System (LVAS) received FDA approval as a bridge-to-transplantation (BTT) treatment option for patients suffering from advanced-stage heart failure.
“THI’s 160-member professional staff has performed more than 100,000 open-heart operations, 200,000 cardiac catheterizations, and 1,000 heart transplants. These record numbers could not have been achieved without the support of more than 10 staff surgeons, 100 surgical residents, cardiologists, radiologists, fellows in training, and devoted nurses and personnel.” Denton A. Cooley
O.H. Frazier, MD, Cardiovascular Surgeon, receives 2008 Ray C. Fish Award in recognition of his contributions to the field of heart transplantation and his pioneering efforts in the research and development of the LVAD.
First clinical trial to treat heart attack patients with a special type of stem cell to promote better healing and to prevent congestive heart failure.
A $2.8 million National Institutes of Health grant to THI funds development of a novel “pulse-less” total artificial heart.
First in Texas to implant a newly FDA-approved, minimally invasive device for the treatment of abdominal aortic aneurysms (AAA).
THI physician scientists provided the impetus for and leadership of one of the largest clinical trials of a cholesterol-lowering drug ever conducted. The JUPITER Trial ended early after producing commanding evidence that elevated CRP blood levels identify patients with increased risk of heart attack, stroke and cardiovascular death, and that treating these individuals with a potent statin markedly reduces their risk.
First in the U.S. to implant a pacemaker enclosed in a mesh envelope embedded with two antibiotic agents that provide site-specific antibiotic protection.
Dr Cooley became president emeritus of THI, and Dr James T. Willerson became president. The fact that Dr Willerson was a cardiologist, not a surgeon, reflected a new emphasis on interventional rath- er than surgical treatment of cardiovascular disease and the growing belief that basic-science breakthroughs, such as regenerative medicine and genetic therapies, offer the best hope for preventing and treating such disease in the future.
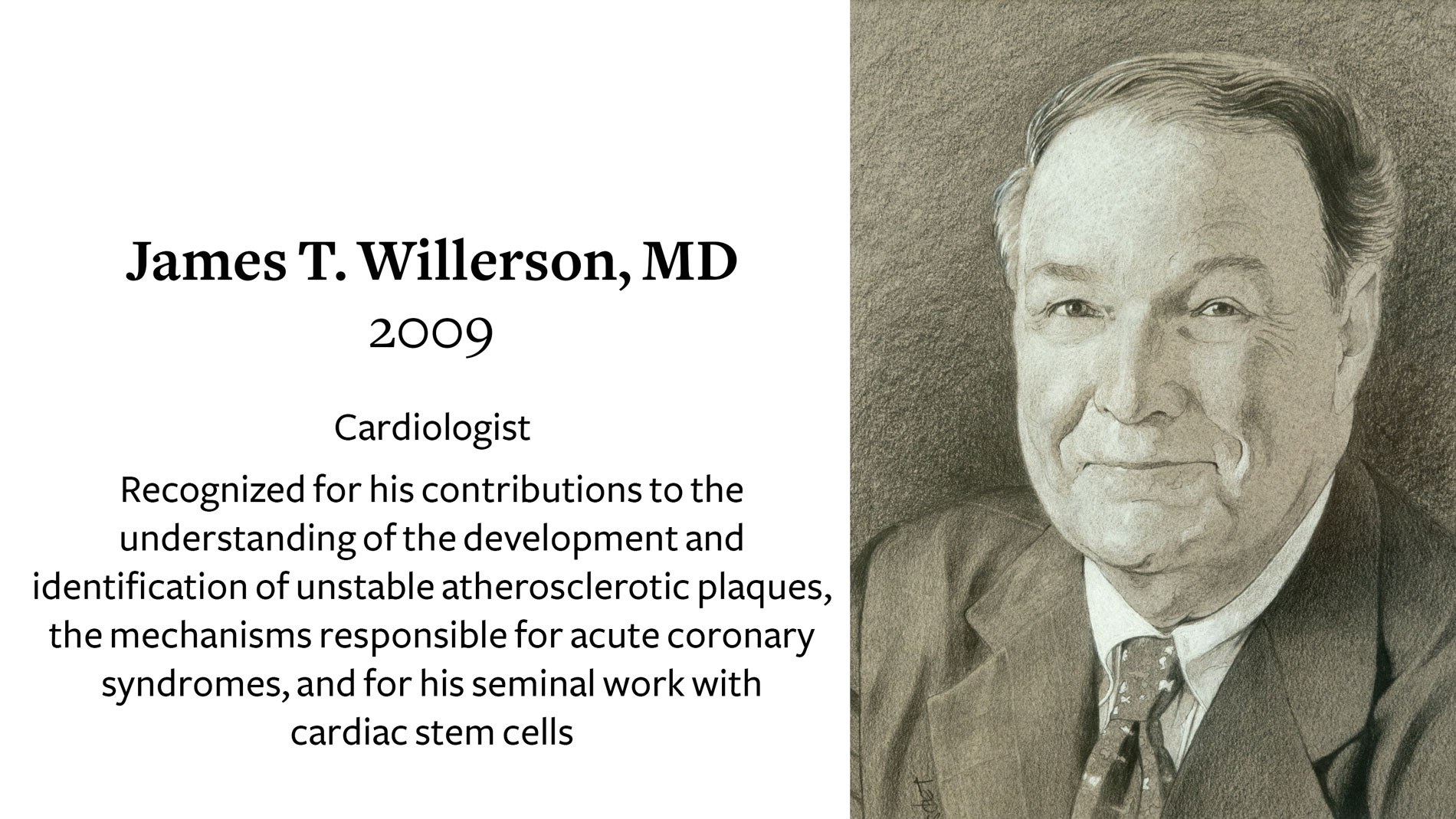
James T. Willerson, MD, receives 2009 Ray C. Fish Award in recognition of his contributions to the understanding of the development and identification of unstable atherosclerotic plaques, the mechanisms responsible for acute coronary syndromes, and for his seminal work with cardiac stem cells
A $4.1 million National Institutes of Health (NIH) grant awarded to THI to determine the effects of a total artificial heart on bodily functions.
$1 million in NIH stimulus funds awarded to THI and Rice University for MRI tracking of stem cells in heart tissue regeneration.
Charles D. Fraser, Jr., MD, Pediatric Cardiovascular Surgeon, receives 2010 Ray C. Fish Award for his program known worldwide for its excellence, its creativity and its ideal outcomes in correcting congenital cardiovascular disease in children.
A $1 million Challenge Grant awarded to THI and University of Houston for research toward developing prostacyclin-secreting cells, a revolutionary cell-based therapy to prevent the effects of Pulmonary Arterial Hypertension (PAH).
FDA approves continuous-flow heart device pioneered at THI for use as a permanent treatment for advanced heart failure.
THI pioneers a breakthrough technique for turning ordinary human skin cells into early-stage heart cells.
THI researchers advance understanding of human development through discovery of stem cell protein regulation.
$1.5 million NIH grant awarded to THI for study of adult stem cells and heart assist devices as combined therapy for heart failure.
Doctors at THI perform carotid artery stent procedure on patient implanted with an LVAD for the first time.
THI and Houston ISD announce medical research program to screen/study 10,000 students/athletes for heart conditions associated with Sudden Cardiac Death.
First in Houston to implant Optical Coherence Tomography (OCT), a new imaging tool to make more informed decisions when assessing and treating heart arterial blockages.
Second in the United States to treat AAA with the minimally invasive Endurant AAA Stent Graft System
THI plays a significant role in clinical studies leading to FDA approval of left-ventricular assist device as a bridge-to-transplantation therapy (HeartMate II Approved as Advanced-Stage Heart Failure Treatment Option )
Patrick W. Serruys, MD, PhD, Interventional Cardiologist, receives 2011 Ray C. Fish Award for major contributions to interventional cardiology recognized worldwide and for the development of a drug-eluting stent.
Dr. Bud Frazier and Dr. Billy Cohn team up with Dr. Daniel Timms, a talented young biomedical engineer from Australia, who has designed a device to support or totally replace the heart. Called the BiVACOR, the machine is small, lightweight and deceptively simple. It uses a magnetic field, a spinning disc and centrifugal force to pump blood.
THI chosen as the Biorepository Core Laboratory for the national Cardiovascular Cell Therapy Research Network and as one of the NIH National Heart, Lung, and Blood Institute (NHLBI) stem cell centers for treating patients with cardiovascular disease with stem cells after a nationwide competition.
Dr. Cooley publishes his memoirs, 100 000 Hearts.
Texas Heart Institute celebrates 30-year anniversary of heart transplantation. More than 1,200 patients have received new hearts, including the longest known survivor who received his heart 29 years ago.
Dr. George J. Reul receives 2012 Ray C. Fish Award, Cardiovascular surgeon honored by Texas Heart Institute for scientific achievements, contributions to cardiology
Texas Heart Institute (THI) and Texas A&M University College of Veterinary Medicine and Biomedical Sciences launch the Center Cell for Cell and Organ Biotechnology
Dr. Alain Cribier, Who Pioneered Heart Valve Replacement with Catheter, Receives Texas Heart Institute’s Ray C. Fish Award for scientific achievement, contributions to cardiology
British Cardiac Pioneer Sir Terence English Receives Texas Heart Institute’s 2014 Ray C. Fish Award for Scientific Achievement, Contributions to Cardiology
One Thousand Left Ventricular Assist Devices: Dr. O.H. “Bud” Frazier implanted the 1000th LVAD at Texas Heart Institute
THI and the University of Texas System renew and expand their legal affiliation
Dr. Delos (Toby) Cosgrove, MD, receives 2016 Ray C. Fish Award
Cardiovascular surgeon and Cleveland Clinic president honored by Texas Heart Institute for scientific achievements, contributions to healthcare
Texas Heart Institute Receives Top Recognition for Continuing Medical Education
Dr. James T. Willerson Named President Emeritus of Texas Heart Institute
Dr. David A. Ott receives 2016 Ray C. Fish Award, Cardiovascular surgeon honored by Texas Heart Institute for contributions to surgery
Texas Heart Institute launches Unparalleled Collaboration to Lead Research on Vascular Diseases in Women
Joseph S. Coselli, MD, the world’s most experienced aortic surgeon, awarded 2018 Ray C Fish Award for Scientific achievement in thoracoabdominal aortic aneurysm repair.
Texas Heart Institute Board Names Emerson Perin, MD, PhD to serve as THI Medical Director.
Emerson Perin, MD, PhD receives 2019 Ray C Fish Award for Scientific achievement for his major contributions to regenerative medicine and stem cell research and developer of novel stem cell approaches to treat patients with ischemic cardiomyopathies and heart failure.
THI Awarded NIH Grant to study a Cardiac Microtissue System to Evaluate and Replicate Clinical Therapy Responses using Patient Cell-Derived Exosomes
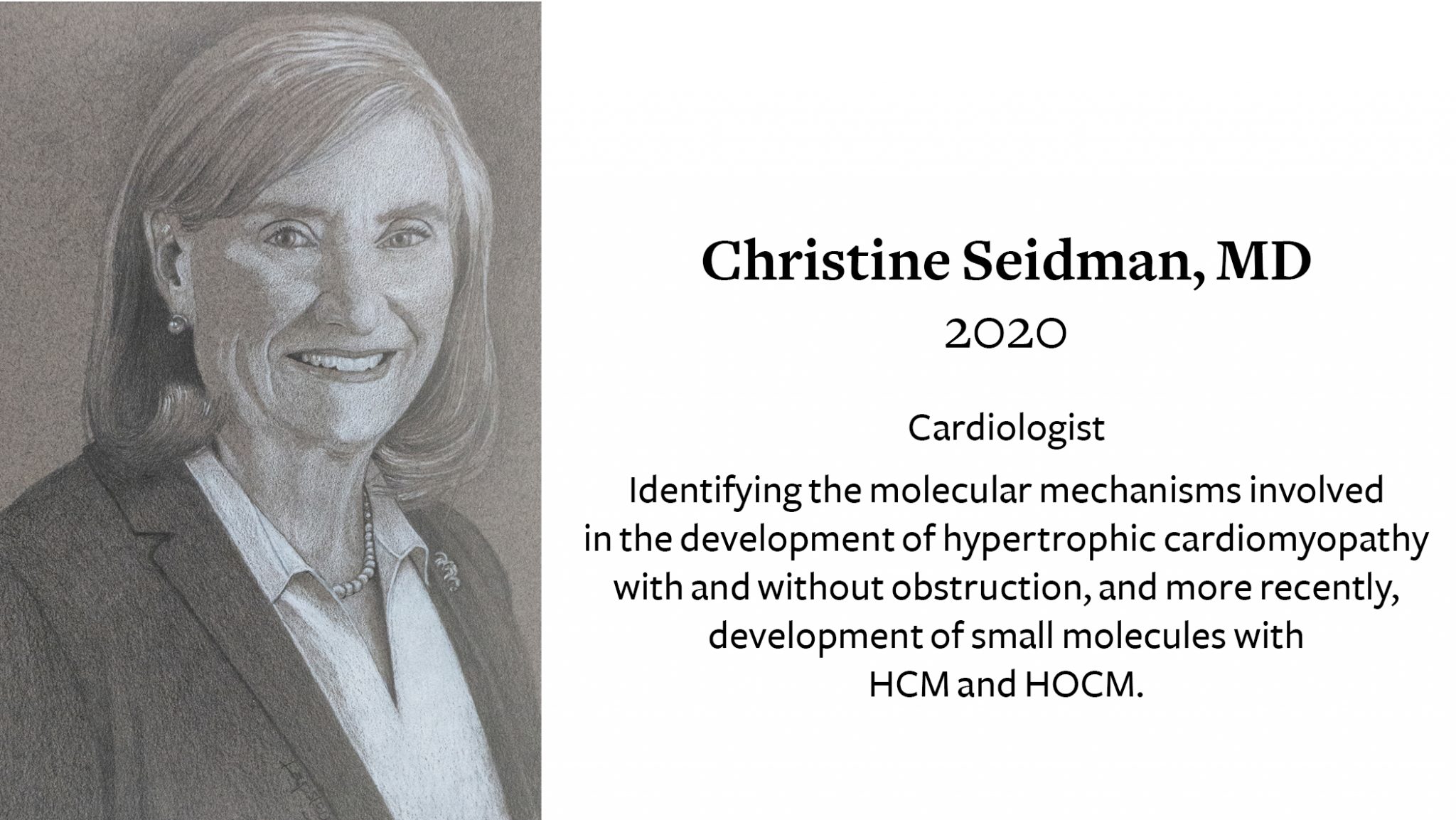
Christine Edry Seidman, MD receives 2020 Ray C. Fish Award for her work identifying and targeting the molecular mechanisms involved in the development of hypertrophic cardiomyopathy. She is the 40th person and the first woman to receive the Texas Heart Institute’s highest honor.
Texas Heart Institute Celebrate’s 30th Year in the U.S. News & World Report Rankings.
As global leaders of patient care for nearly six decades, THI has been ranked among the top cardiovascular centers in the United States by U.S. News & World Report for the past 30 years.
Texas Heart Institute announces its new medical practice: Texas Heart Medical Group. A natural progression of our long-term mission and our commitment to cutting edge cardiac care. Now, we research, teach, and treat—all in one place. Learn More
Seminal research conducted by a team of investigators led by Dr. James Martin published in Science Translational Medicine. A game-changing step forward for the treatment of heart failure using gene therapy to regenerate heart muscle following myocardial infarction.
Texas Heart Institute at Baylor St. Luke’s Medical Center ranked 13th in the nation by U.S. News & World Report. among Adult Cardiology & Heart Surgery hospitals, making it the highest-ranking heart care center in Houston.
THI legend Dr. Bud Frazier won the 2021 Scientific Achievement Award from the American Association for Thoracic Surgery (AATS).
A History of Firsts
Throughout our history, we have been at the forefront of cardiovascular discovery and innovation. In fact, some of the greatest inventions in the history of mankind have been made in Texas — in the heart of the Texas Medical Center, right here at The Texas Heart Institute.
It all started in 1962, when the understanding, diagnosis and treatment of cardiovascular disease were in their infancy. It was then that Dr. Denton A. Cooley made a decision of great importance that would benefit people throughout the state of Texas, across the United States and around the world.
He founded Texas Heart Institute (THI) on August 3, 1962. Its mission: to reduce the devastating toll of cardiovascular disease through innovative programs in research, education and improved patient care.
More than 55 years later, the Institute’s success cannot be counted simply in time, but in new knowledge and discoveries that have advanced the progress against cardiovascular disease, the leading cause of death in the United States. Through our innovative programs in research and education, THI has been the setting of a variety of exciting developments about the causes, prevention, diagnosis and treatment of cardiovascular disease. Among these are the first successful heart transplantation in the U.S., the first implantation of an artificial heart in man in the world, breakthroughs in the treatment of infants born with congenital heart defects, and effective methods of preventing heart attacks by reducing the formation of blockages in the arteries.
In addition, Texas Heart Institute and its clinical partner, Baylor St. Luke’s Medical Center, have become one of the nation’s largest cardiovascular centers. Members of the Institute’s Professional Staff have performed more than 118,800 open heart operations, 258,000 cardiac catheterizations and 1,270 heart transplants – experience no other facility can match.
Donate to Texas Heart Institute
As Dr. Cooley prophesied over half a century ago, Texas Heart Institute has offered hope to victims of cardiovascular disease around the world. As long as heart disease remains a threat to all our lives, our efforts will continue.
Our progress would not be possible without the confidence, commitment and support of our donor community.